By Dr Michael Simmons | GP Longevity Lead at My Wellness Doctor
GLP-1 receptor agonists like semaglutide—marketed under the names; Wegovy & Ozempic—are transforming how doctors manage obesity and diabetes, & their increasing list of health benefits may now also include the treatment of fatty liver disease.

A recent international study suggests semaglutide may significantly improve, and potentially reverse, metabolic dysfunction-associated steatohepatitis (MASH), a progressive form of fatty liver disease.
MASH vs NASH
Previously known as non-alcoholic steatohepatitis (NASH), MASH encompasses the whole spectrum of non-alcoholic fatty liver disease (NAFLD). It’s characterised by hepatic steatosis, lobular inflammation, hepatocellular ballooning, and varying degrees of fibrosis. Left unmanaged, MASH can lead to cirrhosis or hepatocellular carcinoma, especially in people with obesity or type 2 diabetes.
Until now, no licensed pharmacotherapy specifically targeted this condition. Treatment focused on lifestyle modification and comorbidity control. This is where semaglutide may be a game-changer.
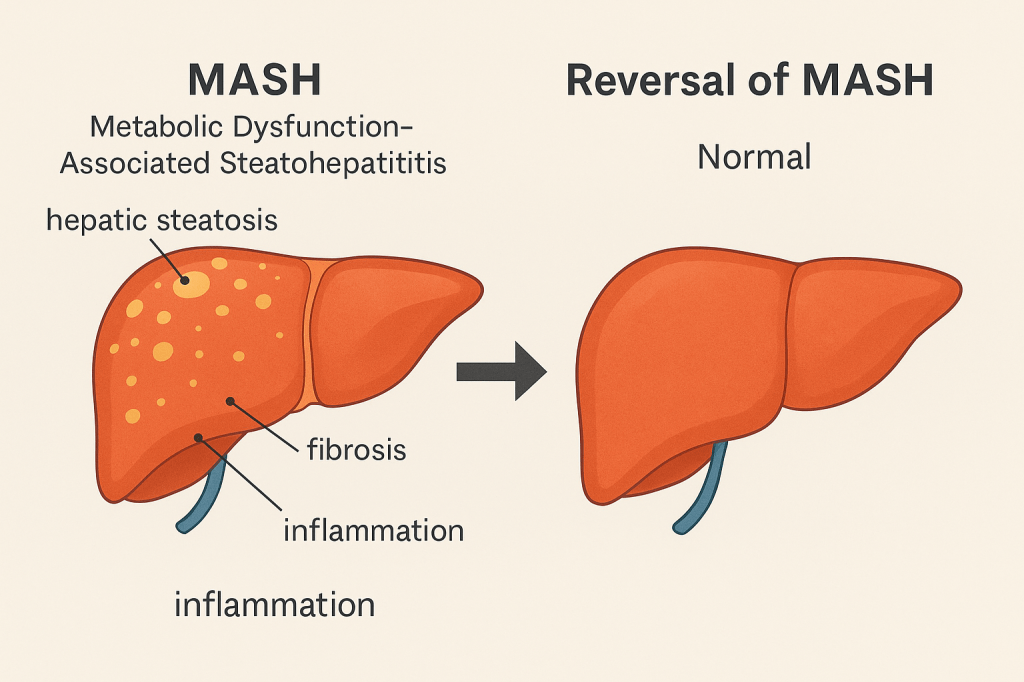
Semaglutide vs MASH
The trial, published in The New England Journal of Medicine, enrolled 1195 patients across 37 countries. Participants received lifestyle counselling and were randomised to weekly semaglutide (up to 2.4 mg, matching the Wegovy protocol) or placebo over a 72-week interim analysis.
Findings:
62.9% of those on semaglutide had marked histological improvements in liver fat and inflammation (vs 34.3% on placebo). 36.8% had fibrosis improvement, compared with 22.4% in the placebo group. Roughly 33% had both outcomes—suggesting disease reversal is potentially achievable.
Importantly, average weight loss in the semaglutide group was 10.5%, versus just 2% in the placebo arm.
Is this all related to weight loss?
While reduced adiposity itself can attenuate hepatic steatosis and inflammation, semaglutide’s effect appears to be due to more than weight loss alone. Early mechanistic data suggest:
1. Reduced hepatic insulin resistance (which underpins Type 2 Diabetes, a driver of fat deposition in the liver) via central and peripheral GLP-1R activation.
2. Direct immunomodulatory action: possibly tempering Kupffer cell and hepatic stellate cell activity. (These cells are normally found in the liver, but become over active in the presence of fatty build up, leading to the changes that drive the progression of MASH).
3. Modulation of inflammatory cytokine signalling, especially in individuals with metabolic syndrome.
It’s plausible that semaglutide improves hepatocellular “microenvironment integrity”—preserving mitochondrial function (cellular energy production), modulating oxidative stress pathways, and reducing hepatocyte apoptosis (cell death).
Clinical Relevance & More Questions
These findings may mark a shift in how we approach metabolic liver disease. For GPs and lifestyle clinicians, this reinforces the idea that targeting metabolic syndrome at its hormonal roots—beyond calorie restriction—can lead to structural reversal of disease.
However, fibrosis regression remained modest overall. Long-term hepatic outcomes (e.g. prevention of cirrhosis or liver cancer) are still unknown. And whether semaglutide offers additional benefit over equivalent weight loss achieved via lifestyle or bariatric intervention is still unclear.
What This Means in Practice
In patients with obesity, diabetes, and suspected or biopsy-proven MASH, semaglutide, & other GLP1 agonists, could become useful new therapies—especially when combined with intensive lifestyle support. For now, it’s not licensed specifically for liver disease, but the evidence base is growing.
For patients seeking meaningful metabolic transformation, this reinforces a key message: improving liver health isn’t just about what we cut out—it’s about how we shift internal signals.
Leave a comment